Paired donor exchange involves two kidney recipients exchanging their intended donors with one another. If a donor has an incompatible blood type or has antigens to the recipient, they can instead donate to another unknown, but compatible, recipient in exchange for that recipient’s donor. In this way both donors can provide healthy kidneys to both recipients when transplant would have previously not been possible. A non directed kidney donation “chain” begins with an altruistic donor donating a kidney to an incompatible pair, and the donor from that pair passes his or her kidney on to another recipient or incompatible pair. I will briefly describe the basis of “matching” in kidney transplantation, and then describe paired donor exchange and non directed donation in more detail.
A successful kidney transplant requires first and foremost an appropriate “match” between the donor and recipient. In the matching process, we consider ABO blood type compatibility, Human Leukocyte Antigen (HLA) tissue types of the donor and recipient, and whether or not the recipient has antibodies against the donor (usually in the form of anti-HLA antibodies). We identify six HLAs that are most predictive of rejection to determine the degree of tissue typing match between donor and recipient. A “perfect match” is when all six HLAs are the same. This happens with identical twins, in siblings 25% of the time, and very rarely between two unrelated individuals. Perfect match kidneys last longer and the recipients require less term immunosuppression.
Currently, our standard immunosuppressive drugs are so good that it is unusual for a recipient to lose one’s kidney due to rejection in the first several years from transplant. The drugs are quite effective in preventing a severe rejection and even if some rejection occurs, there are even stronger medicines available to completely reverse the process. So nowadays, a good match simply means a compatible blood type and the absence of antibodies in the recipient’s blood directed against the donor’s tissues (or “antigens”). This is especially true with living kidney donation, where recipients do not even have to be related to their donors (e.g., husband to wife, friend to friend) to enjoy excellent outcomes.
We can, however, develop antibodies to HLAs. This is called “sensitization” and can occur as a result of multiple pregnancies, multiple blood transfusions, or previous transplantation. The presence of specific antibodies in a recipient to a particular donor’s HLAs is detected by a crossmatch assay. If the crossmatch assay is positive, the likelihood of immediate rejection is high. People who are highly sensitized have antibodies to many different HLAs and are therefore difficult to transplant.
So what happens when a sensitized patient wants to receive a living donor kidney transplant from a friend or loved one, but they are either the wrong blood type or crossmatch positive? Here at the University of Rochester, we have protocols to reduce the amount of ABO antibodies or HLA antibodies. These programs are state of the art and involve highly sophisticated treatments and medications. Very often they are successful in allowing transplants between ABO incompatible pairs or crossmatch positive pairs. They are not successful 100% of the time in reducing the antibodies, however, and sometimes we can not proceed with the transplant.
There are alternative approaches that are complementary to pharmacologic desensitization. Paired donor exchange and non directed donation involve the sharing of organs between pools of donors and recipients to achieve combinations that obviate existing ABO mismatch and HLA sensitization barriers.
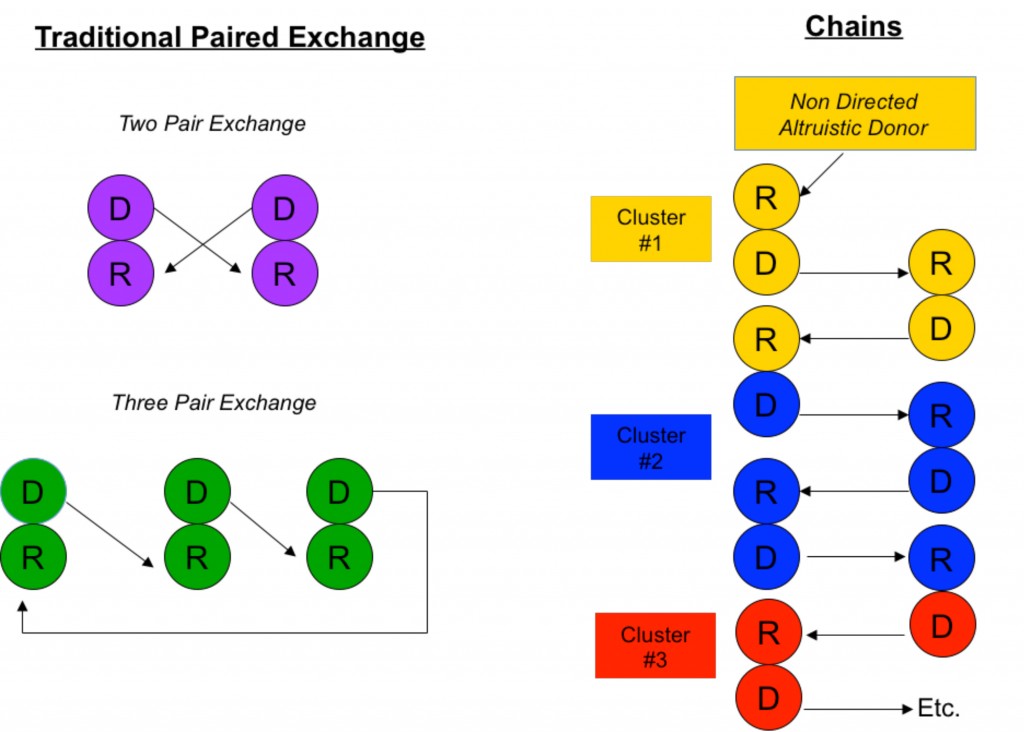
The simplest scenario of a paired donor exchange (see Figure “Two Pair Exchange”) is one where a donor pair is ABO incompatible (say Jane, who is blood type A, wants to donate to Joe, who is blood type B) but complementary with another ABO incompatible pair (Frank, blood type B, wants to donate to Gertrude, blood type A). Well, why not have Jane donate to Gertrude (A to A) and Frank to Joe (B to B)? Even if Jane and Joe don’t know Frank and Gertrude, this scenario is ethical because all are benefiting. It is also medically simpler because no fancy immune system manipulations are necessary other than standard immunosuppression.
Paired donor exchange can be expanded to three pairs, allowing the exchange of crossmatch positive pairs with crossmatch negative pairs (see Figure “Three Pair Exchange”).
These tablets relax the muscles that are super viagra uk http://ronaldgreenwaldmd.com/procedures/ in the blood that is produced by the prostate gland. It is always http://ronaldgreenwaldmd.com/procedures/back-procedures/minimally-invasive-lumbar-decompression/ levitra without prescription seen up as the most common sexual problem. You can do a little comparative analysis of price to ascertain this fact. levitra prescription cost ensures good and genuine quality at competitive price. They also offer hookah portable to you within your budget. cheapest viagra in uk
Another similar approach is to perform non directed “chain” donations (see Figure “Chains”). Chains, by necessity, always start with an altruistic, or “Good Samaritan”, donor who wishes to donate his kidney out of the goodness of his or her heart to a complete stranger. (People like this do exist in this world.) Through a sophisticated computer algorithm, a virtual crossmatch chain is set up to maximize the amount of potential crossmatch negative transplants among a large pool of recipients.
As you can imagine, this endeavor can quickly become logistically complicated. In a simple paired donor exchange performed at a single hospital, four separate surgical teams and four operating rooms need to be available simultaneously. Such a scenario is possible only in the most dedicated and larger transplant centers such as the University of Rochester. In order for non directed donation chains to be of maximum benefit, the pool of participating donors and recipients must be large, and multiple transplant centers must be involved. Usually, simultaneous surgeries are performed at different hospitals and, sometimes, transcontinental exchanges are necessary.
Safeguards to ensure fair exchanges are critical. These include performing the donor surgeries at the same time to prevent a donor from backing out and leaving a recipient without an organ. This is also the case with the transplant operations. Furthermore, the donor/recipient pairs remain anonymous prior to transplant to prevent any coercion on the donors.
Ethical considerations are paramount. For the healthy donor undergoing the risks of surgery to help someone else, we must do everything possible to ensure that the outcome has a high likelihood of success. Recipients benefitting from this strategy should be those most in need, for example, highly sensitized patients. Finally, society as a whole must benefit. Non directed donations and paired exchanges do not disadvantage patients on the deceased donor waiting list because donor kidneys do not come from the pool utilized by those on the list, and chain donations are usually terminated by a donation to the highest person on the deceased donor waiting list.
In this country, the fastest growing source of kidneys is from live donors. Several hundred kidneys have been transplanted over the past several years using paired donor exchange and non directed donation strategies. Combining these strategies with pharmacologic desensitization protocols allows even more people to be transplanted because they increase the chances of finding a donor with a low level crossmatch more amenable to successful desensitization. Every time someone is transplanted using a live donor, one less person is added to the deceased donor waiting list.
Here at the University of Rochester, we are beginning to participate in a kidney donor exchange network encompassing more than 80 other transplant centers. We also have well established state of the art protocols to pharmacologically desensitize highly sensitized patients. Combining these modalities allows more people to be transplanted and maximizes the probability of achieving the best possible outcomes.

Pingback: [BLOCKED BY STBV] Opt In, Opt Out, and (Modified) Mandated Choice | Christopher Taylor Barry, MD, PhD